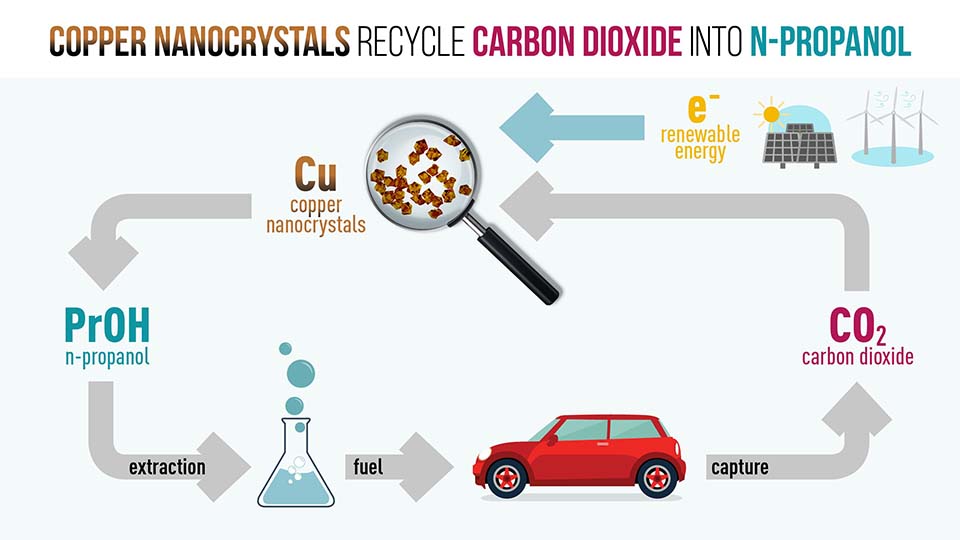
A team led by Assistant Professor Jason Yeo from NUS Chemistry has developed a copper-based catalyst capable of recycling the greenhouse gas carbon dioxide (CO2) into n-propanol, a commercially valuable liquid fuel, in a single step.
Current energy needs are typically met by combusting fossil fuels such as oil, coal and natural gas. However, this is unsustainable since the supply of such fossil fuels is limited and will eventually run out. Burning fossil fuels also generates CO2, a commonly cited culprit in advancing global warming.
Existing methods of reducing CO2 into carbon-based chemicals and fuels are problematic as the gas is highly stable and difficult to activate chemically. Among the alcohols, n-propanol is one of the most difficult and expensive to produce.
To overcome this, Asst Prof Yeo, together with PhD student Ren Dan, used agglomerates of copper nanocrystals — prepared from inexpensive and widely available copper salts — to electrochemically reduce CO2 at room temperature and pressure, and without the use of environmentally harmful organic solvents. The copper nanocrystals yielded 25 times more n-propanol than normal copper nanoparticles and were catalytically stable for at least six hours, with only a 14 per cent deactivation after 12 hours of reduction.
With a very high octane number and energy density, n-propanol commands a higher market value than other CO2 reduction products like methane. It can also be blended with gasoline to deliver cleaner burning fuel with lower greenhouse gas emission, making the discovery a boon for the energy industry.
“This work contributes to a sustainable and green global energy industry, which meets our energy needs while minimising detrimental environmental impact,” said Asst Prof Yeo.