Most of us are aware of the adverse effects of the carbon dioxide (CO2) on the atmosphere. It is the major component of greenhouse gas and causes global warming. It is presumed that the atmospheric CO2concentration will be doubled in the next century. Industrialization is indeed the biggest reason behind this massive CO2emission.Burning of fossil fuels creates a thick black blanket around the earth, increases atmospheric temperature and makes the planet dirty, poisonous and unhealthy for living of plants, animals and humans, which is termed as greenhouse effect.A new threat is generated by this gas inside the ocean, because of the dissolving of the CO2 in ocean water. Life under the ocean is facing major threats.
Ocean acidification terminology implies the reduction of pH of the ocean water for an extended period by absorbing CO2from atmosphere.The complete set of environment change,high acidity,oxygen reduction of ocean is referred to as “Deadly trio”.
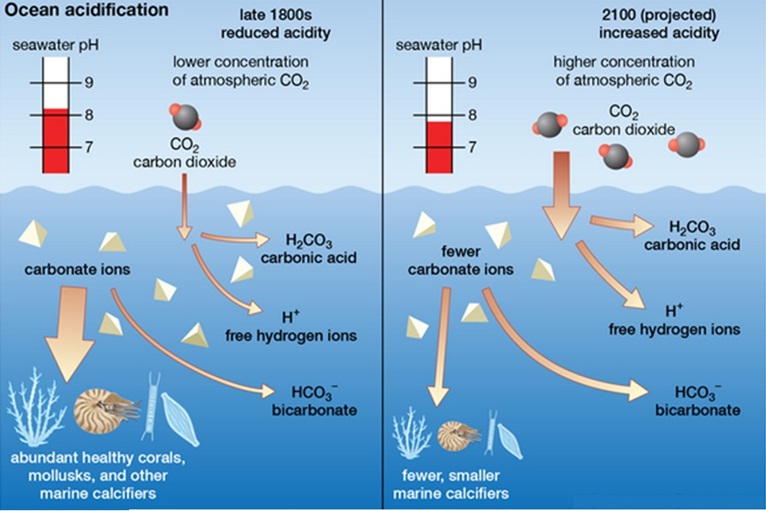
Continuous addition of CO2and other greenhouse gases make a great impact on the biogeochemistry of ocean, which is termed as ocean acidification. From emission scenario, it is known that atmospheric CO2 was increased 400ppm from 280 ppm at preindustrial times and rises 900 ppm from 550 ppm at 21st century. When CO2dissolves in ocean, it lowers the pH and making the ocean more acidic. There is a great impact of ocean acidification on ocean ecosystem and organism.
Every day,Ocean absorbs one third of CO2from human creation and its quantity equals to 22 million tons a day. Recently, researchers started investigation on ocean acidification and it’s impact on marine ecosystem. NOAA's(The National Oceanic and Atmospheric Administration) startedocean acidification program try to monitor the ocean ecosystem by connecting relation among scientists, resource managers, policy makers, and the public.
CO2cycle between atmosphere and ocean water:
A series of chemical reaction is occurred, when CO2is absorbs by sea water. CO2(aq) + H2O ⇌ H2CO3⇌ HCO3− + H+⇌ CO32− + 2 H+.It increases the concentration of H+ ions and reduces the CO32−ion formation.Carbonation is a main building block of maximum calcifying marine zooplankton and organism. Calcium carbonate is the main component of different ocean organism such as corals,sea urchins, oysters, and calcareous plankton,shrimp and lobster etc. pH changes also has a great impact on non-calcifying organism. Due to increase of acidity of water helps to attack predators easily by certain fish that destroys the total ocean food chain.Moreover,whole worlds economic also depend on healthy ocean environment.
It would be perceptible that reduction of pH 0.1 in pH scale is a minor change,but the pH value is calculated in logarithmic scale. Due to logarithmic calculation, acidity of pH 4 is 101 or ten times higher than pH 5 and 102times or hundred times higher than pH 6.From last 20 million years, continue addition of carbon di oxide in a same rate would be a cause of 120 % more acidic ocean water in future, when pH of ocean water will stands on 7.8-7.9 in pH scale.Since the industrialization period, there is a sum of 550 billion of CO2 absorbed by the ocean water from atmosphere, which implies 22 million tons of CO2 per day.
Causes of Ocean Acidification:
- Increase of Carbon IV oxide in ocean:When sea organisms die into ocean, they directly release carbon inside the water
- Increase of carbon IV oxide in air: Continuously a huge amount of carbon dioxide is added in air by different human activities. Sometimes this polluted gas dissolves in water.
- High concentration of H+ ions in water: Some chain reaction occurred on sea bed that makes the water acidic.
- Burning of Fossil fuels:When petroleum,diesel coal product is burnt, it release high amount of CO2 in air and atmospheric pollutant gas enter into sea water.Though it is not directly linked with ocean acidification.
- Disposal of Waste into water: Dangerous liquid from industry,agriculture, domestic area always is added in ocean water, which has directly impact on ocean acidification.
- Industrialization: Harmful gases such as carbon dioxide, sulfur dioxide, nitrogen oxides are emitted from different industry, which are mixed up with air and fall into water as a form of acid rain
Impacts of ocean acidification:
- Some impacts of ocean acidification on marine ecosystem:
An ecosystem is made up of two main biotic component, producer and consumer. The ocean is not the exception of that. In the sea the main two of the main producers are cyanobacteria for shallow sea and archaebacteria for deep sea. The other important productive ecosystem on the ocean is the coral reef. Zooplankton and mussels are primary and secondary consumers. If these organisms die the total ocean ecosystem will fall down and other animals like fish population will be affected. Let’s see the adverse effects of ocean acidification on those organisms.
1. Microbial community:
Archaea are a very important nitrifying organism in the ocean. Their carbon cycle is not a conventional Calvin cycle. They lac RUBISCO, the main enzyme of the calvin cycle. Rather they utilize H2CO3 as a source of carbon for 3 hydroxy propionate/ 4 hydroxybutyrate pathway. In decreased pH the rate of nitrification declines to 20-60%. The decrease nitrification may hamper ocean ecosystem in producer level.
2. Coral reefs:
Coral reefs is called “rainforest of the sea”. Reef is formed by colonization of corals in tropical region. The main component of coral reef is calcium carbonate. It is formed due to deposition of coral polyps and other organism in ocean.Rising acidity in ocean water is a biggest threat to coral reef, which is called “osteoporosis of the sea. By 2080, it is predicted by researcher that high acidity in ocean water can destroy reefs more quickly than formation of reefs.
3. Mussel:
The mussel is the common name used for several members of bivalve mollusks, from saltwater and freshwater habitats. They are found in tidal regions of the coastal zones. Like many creatures in the oceans, which protect themselves with a calcareous shell from predators, the mussel is endangered by the increasing acidification of seawater caused by the uptake of additional carbon dioxide from the atmosphere which is dissolved in seawater. In early life stages, mussels are highly sensitive to decline in pH.
4. Zooplankton:
Zooplankton is a sort of heterotrophic tiny organisms that range from infinitesimal living beings to extensive species, for example, jellyfish. Zooplanktons are found inside extensive water bodies including seas and freshwater frameworks.
There are some zooplankton (Foraminifera, Petropods, Heteropods) which are made of calcium carbonate. Petropods, which is snail like structure is very sensitive to pH change .The picture bellow indicates that shell of petropods dissolve in acidic sea water after 45 days.

Fig 2:Effect of ocean acidification on calcifying organism
They are significant donors of the food web as all bigger life eats zooplankton. They are basic job players in the carbon cycle. At the point when shelled zooplankton dies and sink to the ocean bottom, they convey their calcium carbonate shells with them, which are saved as rock or sediment and put away for the simple future.
These modest living beings recreate so quickly, that they can literally adjust to the expanding corrosiveness, more quickly, than substantial, moderate duplicating life forms. For a miniflora doesn't deal with acidity that well, because of the fact that their shells break down quickly. The shells of pteropods are comprised of aragonite, a sensitive type of calcium carbonate that is 50% more soluble in seawater. Subsequently, pteropods are dissolving in the Southern sea, where more acidic water from the remote ocean ascends to the surface, hurrying the effects of acidification caused by human-generated carbon dioxide.
B. Impact on Food Shortage: Ocean acidification contributes the problem on social economy system. Acidic waters makes the soil acidic, which is not good for agriculture. Besides, acidic water has an important roleon fisheries that is the main source of protein of 1 billion people.
C. Impact on human health:Human uses the water for different purposes. Acidification of water is unhealthy for uses. People eat fish as a protein source. When water acidity is higher, fish consumption would be reason for different diseases and cancer.Baby red king crab cannot survive in acidic water.It provides high quality of protein.So,ocean acidification reduces protein supply to human body.
Solutions:
1. Strict law and regulation: To fight against ocean acidification strict law and regulation should be followed. Scientific waste management process can protect the water body from pollution.
2.Civil Education: Water of ocean is a part of environment.Consevation water body is very important for existence of life. Government and different international organization starts to make awareness program among common people. Education also important to make them understand the rules and regulation.
3.Right sea food consumption: As,Fish grow up in acidic environment, consumption of such fish is not good for health. Before bring them into marketed, hygination taste should be done.
In Box: Global responses to ocean acidification
• National Oceanic and Atmospheric Administration (NOAA) organizes ocean acidification Program which creates awareness in the society about the depriving conditions of the ocean and how to deal with them, through interdisciplinary partnerships, at both national as well as international level
• Global Ocean Acidification Observing Network (GOA-ON) is a communitarian global way to deal with the status and advancement of ocean acidification in vast sea, beachfront and estuarine conditions to comprehend the drivers and effects of ocean acidification on marine biological systems, and to give spatially and transiently settled biogeochemical information important to streamline displaying for ocean acidification.
• UNESCO-IOC and the Scientific Committee on Oceanic Research (SCOR) support the International Ocean Carbon Coordination Project (IOCCP), an observing and research program. IOCCP centersaround the impact of expanding the level of CO2
outflows on the sea and concentrates the impact of ocean acidification on calcifying living beings and coral development rate
Acidification in the Arctic Ocean:
Global warming is the cause of drastic change of Arctic Ocean. Arctic Ocean acidity is rapidly

Fig 3: Graphical representation of ice melting of Antarctic Ocean
Increasing .Sea ice is melted due to decrease the pH. Due to acidity, pacific water is moving towards Arctic Ocean at winter time. Low carbon dioxide helps to intense cooling and photosynthesis of marine organism. Cold water can absorb more carbon dioxide than warm water.It is a major concern for marine life in Arctic Ocean. Though pH change is comparative less in Pacific Ocean than Arctic.
Conclusion: Entire World Ocean is affected by changing of ocean chemistry.Ocean acidification has potential effect on marine ecosystem.Ocean temperature is increased due to pH changes.Temperature changes are directly connected to physiology of marine ecosystem and geographical distribution of species. It threats to food industry,fishing industry and natural shoreline protection. Ocean acidification prevent marine animals to communicate in water by using echolocation.Even, ocean organism collection is very important for resource of biological research,which is alsoaffected due to acidification. Fortunately, there is solution for every problem. The most important solution for protecting our ocean is to reduce CO2footprint.