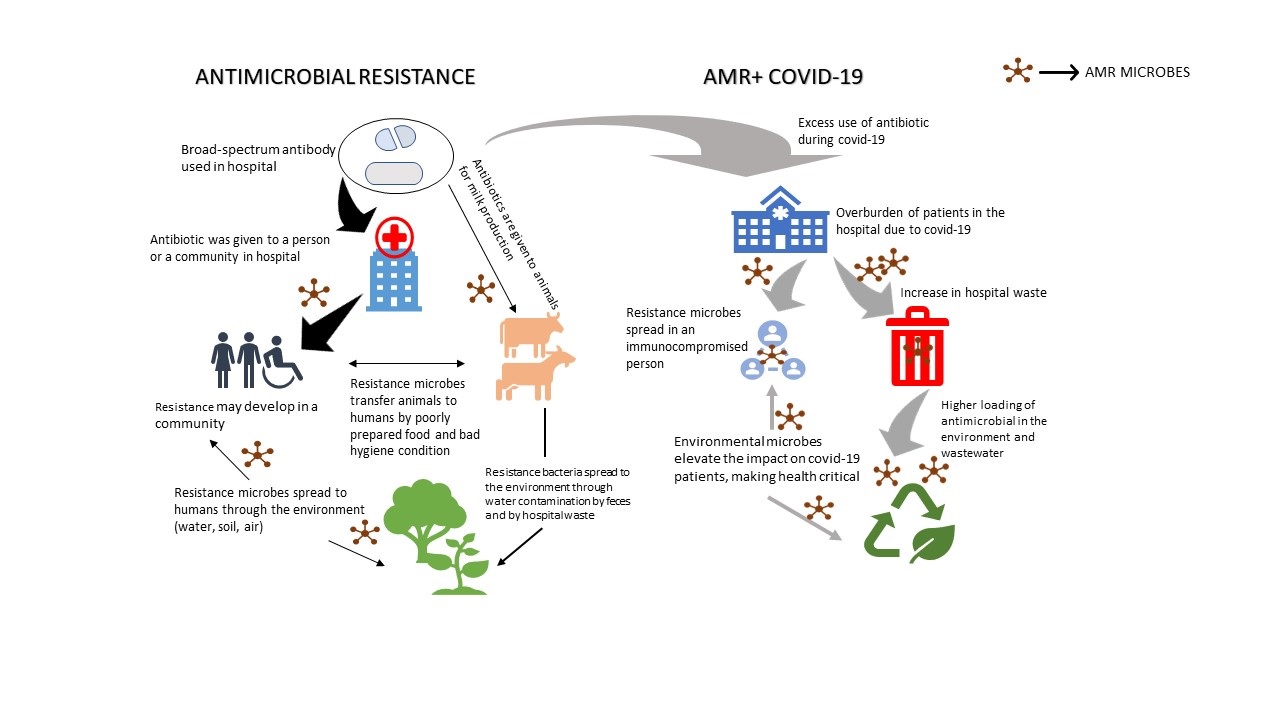
Over the years microorganisms have developed resistance to antibiotics, antifungals, antivirals, antimalarials, and anthelmintics by a phenomenon known as antimicrobial resistance (AMR). Millions of people die each year as a result of lack of comprehensive measures to combat AMR. Most patients in impoverished countries self-treat because either public hospitals are overloaded or would impose higher financial obligations, or they just want to see whether they get better. As a result, the high number of patients who seek antimicrobials directly can have a significant influence on antimicrobial usage rates and AMR development. The substantial rise in wide-ranging antibiotics use in COVID-19-affected nations has put harmful microorganisms under a lot of selection pressure. As a result, in the post-pandemic period, AMR will become a global epidemic in near future.
Excessive usage of disinfectants such as alcohol, quaternary ammonium compounds, phenols, hydrogen peroxide, and solvents, which triggers microbial DNA damage, has risen during COVID-19. The stimulation of translesion synthesis polymerases (TLS) by bacteria in response to DNA damage tolerates and bypasses unrepaired DNA lesions, resulting in mutations that contribute to the development of AMR. According to research, bacterial and fungal infections, some of which are resistant to antibiotics and antifungals, account for roughly half of the deaths of hospitalized COVID-19 patients. Antimicrobial-resistant diseases are most common in healthcare settings like hospitals and nursing homes, where infections can spread quickly among people with weakened immune systems. Some resistant strains viz. Klebsiella pneumonia, Pseudomonas aeruginosa, extended-spectrum beta-lactamase, MDR E. coli, Enterococcus Chlamydia pneumoniae, Mycoplasma pneumoniae, and Acinetobacter have been reported in patients with COVID-19. Additionally, due to differences in healthcare practices, antibiotic medication, and infection prevention techniques, AMR rates would be varied.
COVID-19 consequence on AMR
Three major factors influence the spread of AMR in a community: emergence, transmission, and infection burden at the statistical level. Selective pressures on microbial populations in people, animals, or the environment can cause AMR to arise. Such selection pressures enable resistance acquisition mechanisms such as point mutation or horizontal transfer of genes encoding resistance to one or more antibiotics when they are subjected to the 'drug and bug' of concern. The transfer of these newly emerging antimicrobial-resistant organisms (AROs) between humans, animals, and ecosystems may be enabled or prevented because of environmental circumstances and behaviour. The quantity and variety of infections, as well as the availability, efficacy, and safety of alternate therapies, will determine the severity of ARO-related diseases. Through the direct or indirect effects of pandemic responses, COVID-19 can alter all three of these components. To combat COVID-19, the government has implemented a variety of measures, including domestic and international travel restrictions, school, workplace, and non-essential service closures, physical distancing measures, and mask-wearing. Supply chain interruptions, healthcare access disruptions, financial repercussions, and growing inequality are all consequences of these shifts. To illustrate the way diverse dimensions (Fig.1) of change in response to COVID-19 might alter the onset, transmission, and burden of AMR, we classify these interventions into three aspects (antimicrobial usage, infection prevention, and health system reforms).
Antimicrobial usage
Antimicrobials are given to approximately 70% of COVID-19 patients in either an outpatient or hospital setting. Antimicrobials are prescribed to COVID-19 patients for a variety of reasons. A large cohort of COVID-19 survivors with residual post-COVID-19 chronic lung disease who are at risk of acute symptoms is also likely to emerge because of the pandemic. Long-term outcomes for people who have recovered from moderate to severe COVID-19 are unknown at this time, but high-resolution chest computed tomography has revealed extensive lung damage even several weeks after the illness began. Frequent courses of antibiotics and hospital admissions in patients with post-COVID-19 structural lung damage may increase the risk of colonization and infection with superbugs like Pseudomonas aeruginosa. Antimicrobials have been employed because of their putative direct action on SARS-CoV-2. Bystander selection may result in the establishment of resistance in co-infecting or co-colonizing pathogens. The antimalarial medicine chloroquine, for example, has been used to treat COVID-19 despite accumulating evidence of its ineffectiveness. This is especially concerning in areas with significant non-Plasmodium falciparum malaria incidence, where chloroquine remains the medication of choice for malaria treatment, and its use for COVID-19 may foster the establishment of chloroquine resistance. Because COVID-19 patients might appear with non-specific symptoms (e.g., fever and/or persistent cough), they may be misdiagnosed with other diseases such as malaria or tuberculosis, and vice versa. Depending on the incidence of COVID-19, these overlapping symptoms may result in improper medication, a lack of prescription, and misdiagnosis. As previously stated, this might have an influence on future AMR levels of other diseases. Following the extensive use of azithromycin for WHO-recommended mass medication administration programs for trachoma, the relevance of such bystander selection has already been observed. This is especially important for COVID-19 because azithromycin has been suggested as a possible treatment.
INFECTION PREVENTION
Many of the actions designed to stop SARS-CoV-2 from spreading should also prevent the transmission of antimicrobial-resistant bacteria. It has been suggested that nations with the maximum AMR load are those where disease transmission, rather than antibiotic usage, is the major reason. As a result of enhanced infection prevention control (IPC) as an outcome of COVID-19, AMR prevalence may drop significantly.
The prevalence of infection in hospital environments
Patients with chronic COVID-19 may be admitted to the hospital for extended periods of time, putting them at risk for hospital acquired infections. COVID-19 patients may also need numerous rounds of broad-spectrum antibiotics, mechanical breathing, additional organ support, and/or intrusive devices. This enhances the risk of infection from hospital-associated bacteria such as methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, Candida auris, and Acinetobacter baumannii, which are typically very resistant. Anecdotal evidence from two acute care hospitals in New York and Missouri showed three- to fourfold increase in central line-associated bloodstream infection rates, most likely due to a shift toward a higher-risk case-mix. COVID-19's problems and resource constraints have had an impact on antimicrobial stewardship efforts. Antimicrobial stewardship programs in hospitals are recognized to have the ability to lower the incidence of ARO (antimicrobial resistance microorganisms) infections, with the main goal being to reduce the risk of antibiotic abuse leading to AMR. As a result, COVID-19 may increase the amount of ARO infections directly, with the exact problematic ARO being setting dependent.
Furthermore, fewer patients in secondary care or altered patient characteristics, such as fewer elective surgical operations, might have caused a halt in the transmission of hospital-acquired AROs. In response to the COVID-19 pandemic, improved infection prevention, PPE (personal protective equipment) use, and control measures, as well as targeted hygiene, will help avoid infections in general and hence aid in controlling the development of AMR by preventing ARO transmission and reducing antibiotic prescription for infections. Considering some fears that increased biocide usage might contribute to the development of cross-resistance, the COVID-19 response is likely to prevent infections: Early indicators of a drop in hospital-acquired infection have been observed in English required reporting statistics, but they must be treated with caution due to a decrease in reporting.
Improving hygiene condition
Improper hygiene or sanitation in the household may facilitate the spread of ARO. Hand washing alone has been found in studies to prevent 30 percent of diarrheal episodes in low- and middle-income communities (LMICs). As a result, improved hand hygiene to prevent SARS-CoV-2 transmission might lower diarrhea incidence and hence the large number of antibiotic courses administered to explosive diarrhea children in LMICs (up to 50 percent with diarrhea receive antibiotics). Increased hygiene and food safety precautions in environments like China's wet marketplaces are expected to limit zoonotic ARO transmission. Biocides are anticipated to be used more often in community and clinical settings to promote cleanliness. Low-level usage of these chemicals has been demonstrated to select for resistance to both biocides and antimicrobials, however, the mechanisms and significance of this are driving the spread of AMR to remain unknown.
Physical barriers and travel restrictions
Many infections, including AROs, will be less likely to spread if 'lockdowns' and social or physical distancing measures, such as shutting childcare centers, are used. Invasive bacterial infections, such as Invasive Group a Streptococcal Infections in the United Kingdom, appear to have declined in 2020. This might be connected to a reduction in transmission, albeit reporting difficulties might also be a factor since the majority of the data comes from high-income European countries. Similarly, several settings have witnessed much lower influenza infection rates, which have resulted in a reduction in both unnecessary and reasonable antibiotic use. Physical separation, as well as a greater understanding of infection control measures, may have a long-term effect on ARO, as well as the flu and other respiratory viruses.
Health System Reforms
COVID-19 has uncovered most of the weaknesses in our healthcare systems. In addition to vaccines and new drug, healthcare system should investigate freshly unexplored regulatory approaches to avoid non-widespread pharmaceutical stock losses and supply chain disruptions. There need for a more adaptive healthcare architecture that is more flexible to disruptions has been emphasized during COVID-19 pandemic. Urban communities are also most likely AMR 'hotspots' due to their dense population. More research is required to figure out how to shape our health system low expenses. More research questions that measure and respond to the COVID-19 situation, as well as future antimicrobial stewardship systems are proposed.
Conclusion
More ingenious approaches are urgently needed to promote and encourage the development of new antimicrobials and antiviral drugs for infectious agents, address climate change, improve antimicrobial ownership and control for humans and animals, and integrate health systems with AMR surveillance systems, including close cooperation with drug companies and security agencies and institutions to eliminate the production of deceptive antimicrobial compounds.