The emergence of SARS-COVID -19 pandemic has propelled the biotech industry to accelerate the development of several biological modalities. Some of the medical entities include but not limited to vaccines, nucleic acids, small molecules, convalescent serum, intravenous immunoglobulin (IgG) and monoclonal antibodies (mAbs) or their coformulation. Among them, vaccination is a preferred approach for prevention and treatment of COVID-19 induced disease, however the vaccine evolution is plagued by time constrains and manufacturing hurdles.
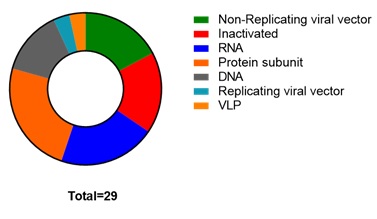
The massive economic and social impact of corona virus has propelled the scientific and government organizations to accelerated the vaccine development. Figure 1 demonstrates multiple platforms of vaccine models targeted against the COVID-19 pandemic causing virus, amid that race two types of vaccine technologies appeared as front runners 1) Non-replicating viral vector (Gene therapy) and 2) Nucleic acid (mRNA) based, where the former delivers the target antigen to the antigen-presenting cells via adenoviral vector while the later via mRNA packed lipid nano-particles.
The vaccine candidates advanced through both platforms express neutralizing antibodies that target the spike protein of the corona virus and inhibits its binding to the human ACE2 protein and prevent infection. ChAd Ox1 viral vector developed by University of Oxford in collaboration with AstraZeneca is the prime example of non-replicating gene therapy-based vaccine, while the Moderna’s mRNA SARS COV-2 vaccine candidate (mRNA-1273) is an exemplary for nucleic acid-based vaccine. Launching a new and novel vaccine through either of the platforms is primarily based on the demonstration of safety, tolerability, efficacy and immunogenicity during phase appropriate clinical studies. The vaccine initiation is also based on the ability to manufacture consistent product through robust process development and validation. Compared to the nucleic acid vaccines, gene therapy vaccine production is afflicted with tedious processes due to required adherence to stringent quality control during the manufacturing of the adenoviral vectors. Figure 2 depicts a comparison of high-level production process for mRNA vaccine Vs. Gene therapy vaccines and appropriate developmental challenges during production process.

Figure 2: Overview of GMP manufacturing process of Non-replicating viral vector and mRNA vaccines
In order to address such challenges, chemistry and manufacturing control (CMC) activities play a vital role. Appropriate physiochemical methods and functional assays are necessary to optimize the quality attributes of the product that determines potency and augmented delivery which is obligatory for appropriate activation of the immune system. This phenomenon is exemplified in the case of gene therapy Covid-19 vaccines, where aggregation or ratio of the empty capsid to the full virus, plays an imperative role in virus titer and potency. In an analogous way fragmentation, encapsulation, and size distribution affects generation of the antibodies by mRNA vaccines. Considering the aggressive timelines to end the pandemic, instead of developing new analytical methodologies, vaccine manufactures can adapt methodologies that are current existent and were previously applied to similar set of modalities in vaccine development.
A number of physiochemical, biophysical, and potency assays were previously developed for Influenza virus, Respiratory Syncytial virus, and Adeno-associated viruses while for mRNA vaccines same methods utilized in siRNA and miRNA analysis could be adapted. For example, Parupudi et al, recently demonstrated comprehensive biophysical characterization of Influenza virions using separation methods Field flow fractionation with multiangle light scattering andAnalytical Ultracentrifugation at different wavelengths (280/260). In this method UV spectrophotometry, Cryoelectron Microscopy, Nanoparticle Tracking Analysis and Dynamic Light Scattering technologies were employed to measure physiochemical properties such as integrity, aggregation &fragmentation, protein/nucleic acid ratio, total particle counts to infectious particles, and morphology. Such methods will assess the overall physiochemical properties along with the manufacturing processes to ensure product safety and consistency. For a protein to be expressed from mRNA, it must have a cap structure, a Poly A tail, and a protein coding region must be accurate and intact. Therefore, mRNA vaccines are tested for those qualities in addition to the purity, size distributions and molecular charge profiles. For the final drug product which is mRNA + Lipid nanoparticle, encapsulation efficiency and surface charge are also tested. Additionally, in the pandemic paradigm, vaccine developers initiate commercial manufacturing before establishing safety and immunogenicity. In such cases, deeper understanding of the process and a thorough vaccine characterization will result in successful scale up of the product, with minimal changes occurring later in the phase 3 of clinical trials.
Further considerations should also be given to large scale process validation, handling during process holding, appropriate temperatures for product storage and supply chain management, which requires dedicated fill finish facilities to avoid potentially hazardous contaminations. The hope is that the large pharmaceutical companies with established technologies, collaborating with contract manufacturing sectors, are well positioned to undertake such challenges.
References:
- Le, T.T et al. Nature Reviews Drug Discovery. 19, 305-306 (2020) The Covid-19Vaccine Development Landscape
- Parupudi, A et al. Journal of Virological Methods. 247, 91-98 (2017) Biophysical Characterization of Influenza Virions