Microbes have been treated since ages with antimicrobial drug which helps in reducing the diseases burden on mankind. However, with continuous usage of drugs microbes such as bacteria, fungi or parasites develops antimicrobial resistance (AMR) and survives in their presence which is a rising obstacle against efficient therapeutics. It is speculated that around 10 million people will die due to AMR by 2050 which will surpass the number of deaths caused due to other reasons.
New drug development apart from being a costly affair requires indefinite time and lots of manpower. Under such pressing scenario utilising the existing drugs against various diseases have recently gained momentum by a phenomenon known as drug repurposing.
Drug repurposing, also known as drug repositioning, is a drug development strategy based on the reuse of existing drugs for new therapeutic usage (Fig. 1). It involves establishing new medical uses for already known drugs, including approved, discontinued, shelved and experimental drugs. In recent years 25% of revenue of the pharmaceutical industry is being generated through drug repurposing. Infact, during the ongoing pandemic, lots of studies were published that described the utility of repurposed drugs when the shortage of drugs threatened the entire world.
Drug repurposing accelerates the traditional drug discovery process by bypassing the requirement for toxicity studies are proven safe and FDA approved. Since drug reuse is based on prior knowledge, such as pharmacokinetic and manufacturing data, the timeline for drug development is greatly shortened, as required investment. The great advantage is that in many cases they have already proven to be safe in pre-clinical models or in the first phase testing in humans. Some studies have suggested that the time of formation may be halved and the costs reduced indeed more – and with a reduced risk of failure. Therefore, they are less likely to fail in terms of safety unless there is an outbreak of drug overdose (Fig. 2).
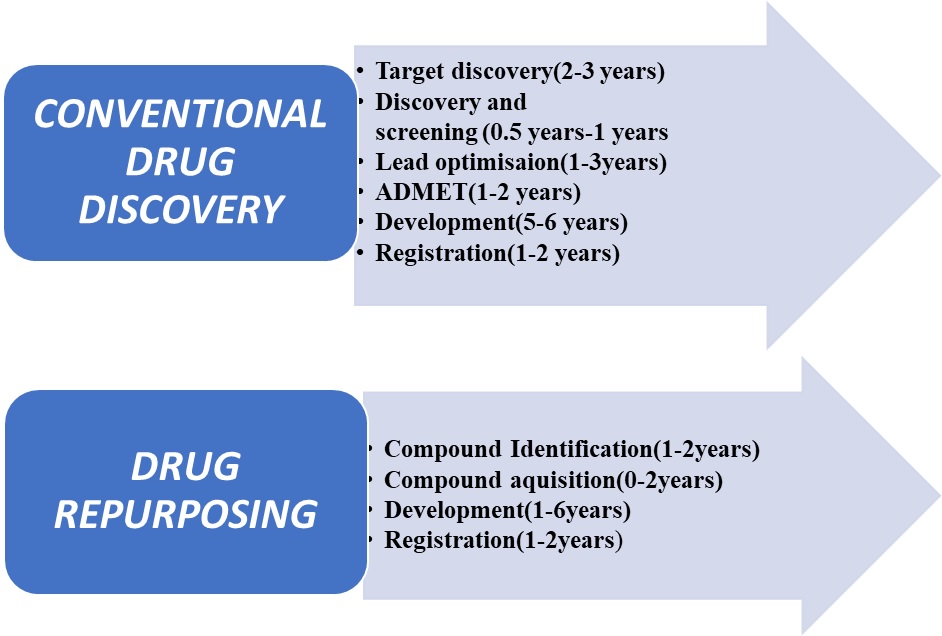
Fig. 2. Differences in steps of conventional drug discovery and drug repurposing
To identify a drug for repurposing, researchers often must analyse many compounds. There are basically two main ways to analyse compounds: computationally and experimentally. In computational methods, researchers analyse large amounts of data such as associated genes, biological pathways, electronic health records, and more to determine potential drug candidates. Moment, with advances in computers and technologies we're fluently suitable to induce massive quantities of data, the challenge is digging the information, interpreting it, and applying the findings. In experimental methods, researchers often physically examine the attributes and/or effects of compounds in a laboratory on disease models There are generally two fundamental drug repositioning principles. First, drugs associated with a selected disease can also work on other diseases thanks to the interdependence between these different diseases. Second, a drug is often related to various targets and pathways since drugs are confounding naturally.
Drug repositioning studies might be classified into two categories supported where the findings originate from drug-based strategies where discovery originates from knowledge associated with drugs (i) disease-based strategies where discovery originates from knowledge related to diseases Although drug repurposing has the potential to decrease the time usually required to reach the market, it is a process that is still associated with many challenges whether from a regulatory board or scientific perspective. May need to fill in the gaps on safety, exposures, & preclinical data on the mechanism of action. Identifying the optimal drug & formulation. Feasibility of clinical trials given unlicensed/off-label access. Existing Intellectual property (IP)/patents on product. Given the high efficacy rates, substantial costs and slow pace of new drug discovery and development, repurposing of 'old' drugs to treat both common and rare diseases is increasingly becoming an attractive proposition to combat AMR because it involves the use of de-risked compounds, with potentially lower overall development costs and shorter development timelines. Together, there is urgency to address the issues related to the overuse and misuse of antimicrobials and prevent from another global pandemic affecting the humankind.