With the emergence of a new infectious strain of the SARS-CoV-2 and the onset of the second wave in India, the respiratory tract infections took a more severe phase. Individuals with its infection have shown damaged lung tissue along with the loss of olfactory and gustatory cell function. Upon exploring the effect of this infection on the microbiome, altered microbiota was observed based on the severity of infection. Gut microbiota plays a vital role in immune response and is also connected with lung tissue via the gut-lung axis. Immunomodulation of gut microbial diversity can prove to be vital in altering the immune response against the SARS-CoV-2 infection, and hence might be beneficial for the COVID infected patients.
Respiratory tract infection is taken into account as a life-threatening disease with over four million annual deaths [1]. Its danger is additionally raised by the occurrence of positive sense, ssRNA virus named SARS CoV-2 and its mutants [2]. Severe infection of this virus leads to renal, hepatic, and gastrointestinal dysfunctions [3] and dysregulation of the immune system [4]. Upon studying genetic homology and pathological features of the infected lungs, the prevalence of cytokine storm is predicted in individuals post severe infection of SARS-CoV- 2 [5]. In the absence of any full proof cure to the infection studies explored every area and optimization of the gut microbial diversity turns out to a potential method of therapy.
Interaction between a coevolving microorganism population also known as Microbiota and the immune system has relevance to human health and disease [6]. The gut microbiota is the main commensal microbial community and features a major role in maintaining homeostasis [7]. Varied studies unconcealed the altered microbiota composition in patients with SARS-CoV-2 infection/COVID 19 [8, 9]. The altered microbiota in relation to COVID-19 can be further supported by the fact that it is highly fatal in elderly people which has decreased the diversity of gut microbiota. Probiotics are known to counter alterations like these. It is believed that probiotics balancing the immune system and microbiome interactions may provide resistance against various pathogens [10]. Therefore, this study/article will give you an insight into the role of probiotics in various respiratory infections and how they are helpful against SARS CoV-2 infection.
1. INTERACTION OF SARS-CoV-2 AND GUT MICROBIOME
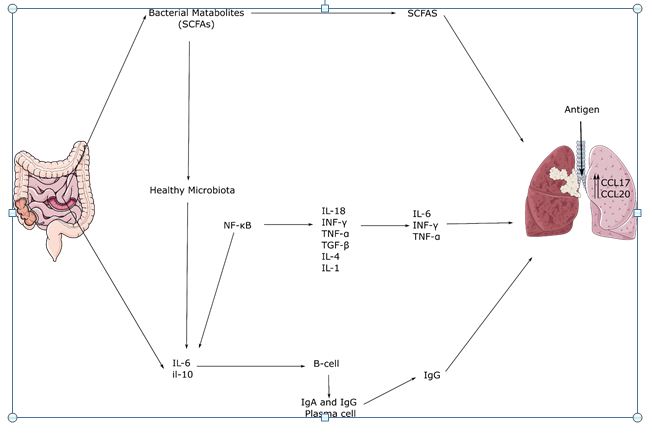
Gut microbiota has an intensive role in various lung diseases through the gut-lung axis – a bidirectional communication system. Gut dysbiosis results in the breakdown of epithelium and inflammation which in turn increases the level of the preferred target of SARS CoV-2 Angiotensin-converting enzyme 2 (ACE 2). Apart from this triggering of inflammatory cascade occurs as a result of the leak and systemic circulation of pro-inflammatory bacterial products [11]. It is seen that SARS CoV-2 infection lowers the microbial diversity and decreases the levels of major beneficial bacteria like Lactobacillus and Bifidobacterium [12]. Individuals with SARS CoV-2 infection also showed the diminished expression of varied gut commensals like Faecalibacterium prausnitzii, Eubacterium rectale, and bifidobacteria, and Bifidobacteria have immunomodulatory potential. Microorganisms distinguishing COVID-19 patients with healthy individuals are Fusicatenibacter, Romboutsia, Intestinibacter, Actinomyces, and Erysipela Clostridium [13]. The altercation and loss of function of the gut microbiome are even confirmed by using a non-human primate animal model (the macaque).
2. GUT-LUNG AXIS AND MICROBIOME
The gut-lung axis is a conceptual bidirectional interaction between the lungs and the intestinal tract, positing that modulation of the gut microbiome has an impact on the lungs and related diseases [14]. It has already been reported that the SARS-CoV-2 infection generally lasts longer in patients with a history of gastrointestinal problems [15]. COVID patients showed overall decreased levels of the gut bacterias like Lactobacillus and Bifidobacterium, along with a relatively higher abundance of pathogenic species like Streptococcus spp., Rothia spp., Veillonella spp., and Actinomyces spp [12]. The COVID-19-related gastrointestinal symptoms may be explained by apoptosis failure in the intestine tract linked to subsequent respiratory infections [16]. Gut microbes show significant microbial inhibitory activities for the lung tissues, with the help of NK cells, neutrophils, and alveolar macrophages [17,18]. Bacterial metabolites, such as short-chain fatty acids (SCFAs), have been shown to reduce inflammatory responses in the lungs to a greater degree. Furthermore, the production of various antiviral molecules including the nuclear factor erythroid 2p45-related factor 2 (Nrf2) and its target Heme oxygenase-1 (HO-1) can be boosted with the help of certain bacterial strains [19]. Bacteriotherapy can reduce gastrointestinal symptoms and protect the respiratory tract, given the microbiome’s important role in modulating the immune and inflammatory responses of the host.
3. MICROBIOME BASED IMMUNOMODULATION
The host’s relationships with the microbiota are intricate, multiple, and bidirectional. The gut microbiota is considered to play a key role in the innate and adaptive immune systems’ function and development [20,21]. Several research and studies showed that particular microbial strains in the gut facilitate the prevention of viral infections such as sepsis, gastroenteritis, and respiratory tract syndrome, improving the epithelial barrier and competing with pathogens for adherence and nutrients [22]. SARS-CoV-2 interacts with ACE2 receptors in the gut and lungs for cellular invasion; it is suggested that ACE inhibitors might reduce pulmonary inflammation and benefit the COVID patients [23]. Bioactive peptides released during food fermentation may act as ACE inhibitors due to their property of binding to binding sites of ACE enzymes. Thus, the gut microbiome can be modulated to alter the immune response in the lung tissue and can play a vital role in the treatment of COVID-19 infected patients or can reduce the impact to a greater extent.
4. PROBIOTICS A USEFUL TREATMENT FOR SARS-CoV-2
According to the UN’s Food and Agriculture Organization (FAO) and the World Health Organization (WHO) microorganisms that can have a positive influence on health are known as probiotics. They are known to facilitate pathogen tolerance by balancing the relationship between the host gut microbiota and the immune system [24,25]. Probiotics have been shown to minimize the severity of acute upper respiratory tract infections (URTIs) hence, are suggested as a treatment option for various viral respiratory infections.[26]. Improving gut microbiota profile by improved diet and nutrition has been shown to boost immunity, and may be one of the preventative strategies for reducing disease symptoms. Further research shows functional diets, such as prebiotics and probiotics, integrated with existing therapies can enhance gut microbiota functioning and reduce the severity of the viral infection [20]. Some example includes Faecalibacterium prausnitzii, Akkermansia muciniphila, Clostridium butyricum, are among the probiotics that inhibit inflammation by decreasing inflammatory cytokines (tumor necrosis factor α (TNFα), and the interleukins, IL-1B, IL-6, and IL-8) and by increasing interleukin-10 (IL-10). Of these, A. muciniphila also restores healthy microbiota. Alongside these microorganisms’ bacterial metabolites like DAT(Desaminotyrosine), butyrate, etc also show anti-viral properties and may help in the therapy of respiratory tract infection [24].
5. CONCLUSION
The interaction of SARS-CoV-2 with gut microbiota composition indicates that microbes play a significant role in the severity of the viral infection by influencing the host immune response against the SARS-CoV-2 infection. Hypersensitivity, susceptibility, and systemic inflammation for the infection may all be caused by dysfunction of the gut microbiota in hosts. Gut dysbiosis results in the breakdown of epithelium and inflammation which in turn increases the level of the preferred target of SARS CoV-2 – Angiotensin-converting enzyme 2(ACE 2). Apart from this, the triggering of the inflammatory cascade occurs as a result of the leak and systemic circulation of pro-inflammatory bacterial products. Hence altering the gut microbiome for targeting the response against SARS-CoV-2 infection seems to be a viable option. The gut microbiome can be increased in population or can be used to produce vital metabolites that can be beneficial for the health and overall well-being of the infected patients. As diet plays a significant role in regulation or dysregulation and its pathological effect so customized functional foods, including prebiotics and probiotics which have the ability to strengthen the microbiota, should be taken under consideration as a novel approach for modulation of microbes and a rehabilitation route for SARS-CoV-2.
REFERENCES
- Ferkol, T., & Schraufnagel, D. (2014). The global burden of respiratory disease. Annals of the American Thoracic Society, 11(3), 404-406.
- Zhu, N., Zhang, D., Wang, W., Li, X., Yang, B., Song, J., … & Tan, W. (2020). A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of medicine.
- Gautier, T., Gall, D. L., Sweidan, A., Tamanai-Shacoori, Z., Jolivet-Gougeon, A., Loréal, O., & Bousarghin, L. (2021). Next-Generation Probiotics and Their Metabolites in COVID-19. Microorganisms, 9(5), 941.
- Qin, C., Zhou, L., Hu, Z., Zhang, S., Yang, S., Tao, Y., … & Tian, D. S. (2020). Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clinical infectious diseases, 71(15), 762-768.
- Zhang, R., Wang, X., Ni, L., Di, X., Ma, B., Niu, S., … & Reiter, R. J. (2020). COVID-19: Melatonin as a potential adjuvant treatment. Life sciences, 250, 117583.
- Ahmadi Badi, S., Tarashi, S., Fateh, A., Rohani, P., Masotti, A., & Siadat, S. D. (2021). From the Role of Microbiota in Gut-Lung Axis to SARS-CoV-2 Pathogenesis. Mediators of Inflammation, 2021.
- Flint, H. J., Duncan, S. H., Scott, K. P., & Louis, P. (2015). Links between diet, gut microbiota composition and gut metabolism. Proceedings of the Nutrition Society, 74(1), 13-22.
- Gou, W., Fu, Y., Yue, L., Chen, G. D., Cai, X., Shuai, M., … & Zheng, J. S. (2020). Gut microbiota may underlie the predisposition of healthy individuals to COVID-19. MedRxiv.
- Kruglikov, I. L., Shah, M., & Scherer, P. E. (2020). Obesity and diabetes as comorbidities for COVID-19: Underlying mechanisms and the role of viral–bacterial interactions. Elife, 9, e61330.
- Lee, E. S., Song, E. J., Nam, Y. D., & Lee, S. Y. (2018). Probiotics in human health and disease: from nutribiotics to pharmabiotics. Journal of Microbiology, 56(11), 773-782.Lee, E. S., Song, E. J., Nam, Y. D., & Lee, S. Y. (2018). Probiotics in human health and disease: from nutribiotics to pharmabiotics. Journal of Microbiology, 56(11), 773-782.
- Thevaranjan, N., Puchta, A., Schulz, C., Naidoo, A., Szamosi, J. C., Verschoor, C. P., … & Bowdish, D. M. (2017). Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell host & microbe, 21(4), 455-466.
- Baud, D., Dimopoulou Agri, V., Gibson, G. R., Reid, G., & Giannoni, E. (2020). Using probiotics to flatten the curve of coronavirus disease COVID-2019 pandemic. Frontiers in public health, 8, 186.
- Segal, J. P., Mak, J. W., Mullish, B. H., Alexander, J. L., Ng, S. C., & Marchesi, J. R. (2020). The gut microbiome: an under-recognised contributor to the COVID-19 pandemic?. Therapeutic Advances in Gastroenterology, 13, 1756284820974914.
- Tulic MK, Piche T, Verhasselt V. Lunggut cross-talk: evidence, mechanisms and implications for the mucosal inflammatory diseases. Clin Exp Allergy. 2016;46:519–28.
- Nobel YR, Phipps M, Zucker J, Lebwohl B, Wang TC, Sobieszczyk ME, et al. Gastrointestinal symptoms and COVID-19: case-control study from the United States. Gastroenterology. 2020 Jul;159((1)):373–5.e2
- Perrone EE, Jung E, Breed E, Dominguez JA, Liang Z, Clark AT, et al. Mechanisms of methicillin-resistant staphylococcus aureus pneumonia-induced intestinal epithelial apoptosis. Shock. 2012 Jul;38((1)):68–75.
- Vieira AT, Rocha VM, Tavares L, Garcia CC, Teixeira MM, Oliveira SC, et al. Control of Klebsiella pneumoniae pulmonary infection and immunomodulation by oral treatment with the commensal probiotic Bifidobacterium longum 5(1A) Microbes Infect. 2016;18:180–9.
- 33. Belkacem N, Serafini N, Wheeler R, Derrien M, Boucinha L, Couesnon A, et al. Lactobacillus paracasei feeding improves immune control of influenza infection in mice. PLoS One. 2017;12((9)):e0184976.
- Spagnolello O, Pinacchio C, Santinelli L, Vassalini P, Innocenti GP, De Girolamo G, Fabris S, Giovanetti M, Angeletti S, Russo A, Mastroianni CM, Ciccozzi M, Ceccarelli G, d’Ettorre G. Targeting Microbiome: An Alternative Strategy for Fighting SARS-CoV-2 Infection. Chemotherapy. 2021 Mar 23:1-9. doi: 10.1159/000515344. Epub ahead of print. PMID: 33756475; PMCID: PMC8089442.
- Dhar D, Mohanty A. Gut microbiota and Covid-19- possible link and implications. Virus Res. 2020 Aug;285:198018. doi: 10.1016/j.virusres.2020.198018. Epub 2020 May 13. PMID: 32430279; PMCID: PMC7217790.
- Negi S, Das DK, Pahari S, Nadeem S, Agrewala JN. Potential Role of Gut Microbiota in Induction and Regulation of Innate Immune Memory. Front Immunol. 2019 Oct 25;10:2441. doi: 10.3389/fimmu.2019.02441. PMID: 31749793; PMCID: PMC6842962.
- Lin L, Jiang X, Zhang Z, Huang S, Zhang Z, Fang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69((6)):997–1001.
- Meng J, Xiao G, Zhang J, He X, Ou M, Bi J, et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9((1)):757–60.
- Gautier, T.; David-Le Gall, S.; Sweidan, A.; Tamanai-Shacoori, Z.; Jolivet-Gougeon, A.; Loréal, O.; Bousarghin, L. Next-Generation Probiotics and Their Metabolites in COVID-19. Microorganisms 2021, 9, 941. https://doi.org/10.3390/microorganisms9050941
- Lee ES, Song EJ, Nam YD, Lee SY. Probiotics in human health and disease: from nutribiotics to pharmabiotics. J Microbiol. 2018 Nov;56(11):773-782. doi: 10.1007/s12275-018-8293-y. Epub 2018 Oct 24. PMID: 30353462.
- Hao Q, Dong BR, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2015 Feb 3;(2):CD006895. doi: 10.1002/14651858.CD006895.pub3. PMID: 25927096.