Several ayurvedic plants have been reported to possess cancer preventive and chemotherapeutic potential. Recent studies have shown how phytochemicals in common Ayurvedic plants inhibit cancer progression by targeting different molecular pathways. Ashwagandha (Withania somnifera), one such medicinal plant, has been investigated for its anticancer potential and mechanisms of action.
Due to the presence of active substances like withaferin A, ashwagandha can inhibit various cancer cell lines by targeting cellular proteins involved in tumor progression, metastasis, cell cycle progression, cell division, and telomere shortening. Its antiproliferative efficacy can be enhanced further via coating gold nanoparticles with its polyphenols.
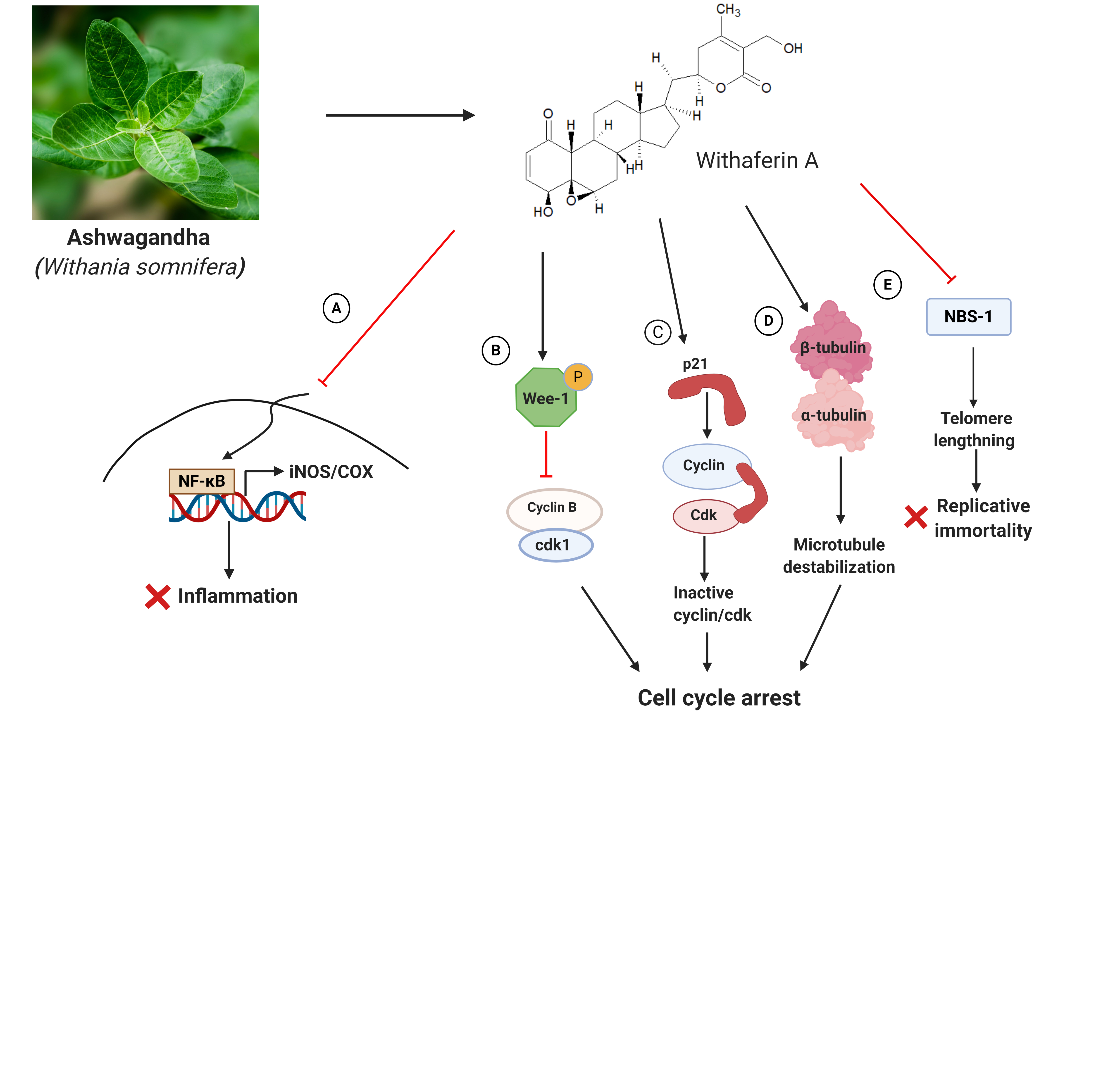
Ashwagandha, an evergreen plant from Solanaceae family, is known for its medicinal properties. It is an ingredient in several traditional medicines where it has been used for the treatment of rheumatism, leukoderma, constipation, insomnia, goitre, and chronic stress [1]. Ashwagandha has also been investigated extensively for its anticancer properties [2]. The therapeutic properties of this shrub are due to the presence of bioactive molecules including withaferin A, withanone, and triethylene glycol [1]. It exerts its anticancer action by preventing inflammation and inhibiting cell division. The enzymes COX (for cyclooxygenase) and iNOS (for inducible nitric oxide synthase) are responsible for inducing a cascade of pro-inflammatory pathways that can eventually lead to inflammation of tumor microenvironment and consequently to the tumor progression and metastasis [3]. Withaferin A inhibits iNOS and COX by preventing the activation of NF-κB in this pathway [4] (Fig. 1A). In addition to its ability to inhibit cancer progression by modulating pro-inflammatory pathways, withaferin-A also promotes cell cycle arrest. It halts cell cycle progression via modulation of key cell cycle-associated proteins such as Wee-1 (Fig. 1B), and p21 (Fig. 1C) [5]. A predominant target of withaferin A is tubulin. Tubulin is a dimeric protein that forms the building block of the cytoskeletal filament, microtubules. Microtubules orchestrate cell division by building the mitotic spindle to organize and segregate duplicated chromosomes. Any disturbance in the structure of this dynamic filament can halt cell cycle and promote cell death. Withaferin A downregulates the expression of tubulin in cancer cells [6]. Further, by binding to the protein at cysteine 239, it perturbs the assembly dynamics of microtubules [7] (Fig. 1D), facilitating cell cycle arrest.
Cancer cells adopt several strategies for their survival. One such tactic is escaping telomere shortening [8] as continued shortening of telomere in successive cell divisions eventually retards the division potential of the cell. To counteract telomere shortening, a mechanism, called ALT (for alternative lengthening of telomeres), operates with high efficiency in many types of tumor cells [9]. ALT is under the care of the protein factors, TRF2 (for telomeric repeat factor-2), POT1 (for protection of telomeric protein-1) and the MRN (MRE11-RAD50-NBS1) complex protein, NBS-1 [10] (Fig. 1E). These proteins are sometimes referred as sheltering complex proteins. Withaferin-A suppresses cancer cell proliferation by suppressing NBS-1 [10].
Although the polyphenols of ashwagandha have been well characterized for their chemical and biological attributes, an effective mechanism of their collective and selective delivery to tumor cells is required. Such a system would enhance the anticancer efficacy of these polyphenols while mitigating potential off-target side effects. One approach to tackle this problem is to coat these polyphenols on size- and shape-optimized gold nanoparticles. It has been shown recently that such fabrication is feasible and that Ashwagandha -coated gold nanoparticles (Ws-GNPs) are potent inhibitors of cancer cell proliferation [11]. How does gold help here? Gold nanoparticles have been shown to accumulate at the site of tumor due to its leaky vasculature and defective lymphatic system [12]. Moreover, selectivity of Ws-GNPs to specific type of tumor can be further enhanced by conjugating with tumor-specific antibodies.
Figure 1: Anticancer mechanism of Ashwagandha with respect to Withaferin A. Withaferin A prevents cancer-causing/promoting inflammation by preventing the transcription of iNOS and COX2 through inhibition of NF-κB (A). Further, by enhancing phosphorylated Wee-1 (B), and p21 (C), Withaferin A arrests cell cycle progression. By targeting tubulin, it further facilitates cell cycle arrest (D). It inhibits the transcription of the telomere-protecting sheltering complex protein, NBS-1, to counteract the replicative vigour of cancer cells (E).
Acknowledgement
The authors thank UM-DAE Centre for Excellence in Basic Sciences for financial support. The figure used in this article was created using a paid version of BioRender software.
Further Reading:
- Singh N, et al. An overview on Ashwagandha: A Rasayana (Rejuvenator) of Ayurveda. African J Tradit Complement Altern Med. 2011;8(5suppl):208-213. doi:10.4314/ajtcam.v8i5S.9
- Wadhwa R, et al. Water Extract of Ashwagandha Leaves Has Anticancer Activity: Identification of an Active Component and Its Mechanism of Action. PLoS One. 2013;8(10):1-11. doi:10.1371/journal.pone.0077189
- Kundu JK, Surh YJ. Inflammation: Gearing the journey to cancer. Mutat Res. 2008;659(1-2):15-30. doi:10.1016/j.mrrev.2008.03.002
- Heyninck K, et al. Withaferin A inhibits NF-kappaB activation by targeting cysteine 179 in IKKβ. Biochem Pharmacol. 2014;91(4):501-509. doi:10.1016/j.bcp.2014.08.004
- Ram V Roy, et al. Withaferin-A induces mitotic catastrophe and growth arrest in prostate cancer cells. J Nat Prod. 2013;76(10):1909–1915. doi:10.1021/np400441f
- Antony ML, et al. Growth arrest by the antitumor steroidal lactone withaferin a in human breast cancer cells is associated with down-regulation and covalent binding at cysteine 303 of β-tubulin. J Biol Chem. 2014;289(3):1852-1865. doi:10.1074/jbc.M113.496844
- Yang J, et al. The natural compound withaferin A covalently binds to Cys239 of β-tubulin to promote tubulin degradation. Mol Pharmacol. 2019;96(6):711-719. doi:10.1124/mol.119.117812
- Okamoto K, Seimiya H. Revisiting Telomere Shortening in Cancer. Cells. 2019;8(2):107. doi:10.3390/cells8020107
- Reddel RR, et al. Alternative Lengthening of Telomeres in Human Cells. Radiat Res. 2001;155(1):194-200. doi:10.1667/0033-7587(2001)155[0194:alotih]2.0.co;2
- Yu Y, et al. Withaferin-A kills cancer cells with and without telomerase: Chemical, computational and experimental evidences. Cell Death Dis. 2017;8(4):e2755. doi:10.1038/cddis.2017.33
- K Meher, et al., Ashwagandha-polyphenols-functionalized gold nanoparticles facilitate apoptosis by perturbing microtubule assembly dynamics in breast cancer cells, J Drug Deliv Sci Technol. 2022;70: 1773-2247. Doi:10.1016/j.jddst.2022.103225
- Golombek SK, et al. Tumor targeting via EPR: Strategies to enhance patient responses. Adv Drug Deliv Rev. 2018;130:17-38. doi:10.1016/j.addr.2018.07.007