The ever-increasing demand of food production to feed a rapid growing population has put a lot of pressure on our crop fields. The world population is expected to reach 10 billion by 2050 and providing food to such a large population is a major challenge. The growth and productivity of crop is highly affected by various biotic and abiotic factors including, high temperature, salt, drought, cold, pathogens and diseases.
Therefore, the immediate need of hour is to introduce some alternate strategies that could help to improve the food production and accelerate sustainable agriculture development.
With the advancement in technology, various techniques of plant genetic engineering to improve the crop yields have been discovered. One of the most recent and promising approaches that has come into lime light is Genome Editing. Genome editing is also known as ‘Gene Editing’, comprises of several techniques which enables the scientists to modify the genome of a plant, resulting in desired trait in a crop. Genome editing is a simple, precise and powerful tool that has given hope to scientists to develop improved crop varieties with higher yields, reduce input costs, stress tolerance and high nutritional values. Gene editing can be implied in a number of ways, either by altering the few nucleotides among the billions found in the genomes, by altering the full allele or by inserting the desired gene in pre-determined regions in the genome. Gene editing is quite precise than any other kind of transgenic or conventional breeding approaches. Thus, this technology is hopefully capable of securing the world’s food supply. Genome editing not only enhances the crop nutritional values but also aids to confer the tolerance in plants against various pests and pathogens and other abiotic factors. Crops that are developed using gene editing technique could avoid those stringent regulation procedures that are related with the GM (genetically modified) crop development. Hence, this made the scientists to believe that the crops developed through precise and specific gene editing techniques would be more acceptable to the common masses than the transgenic plants. Moreover, the product is labelled as ‘non-GMO’ because of the absence of any kind of foreign DNA. It can be carried out in any laboratory and even with the crops that have complex genetic material and are not easy to breed using conventional approach.
After that, Zinc finger nucleases (ZFN) were introduced as the first programmable enzyme for genome editing. ZFNs are basically some fused proteins that comprise of DNA binding domain. They make site-specific double-stranded breaks by binding to specific 3 - 4 base pair (bp) long sequences. ZFNs remained the only site-specific gene editing enzyme for many years until the discovery of transcription activator-like nucleases (TALENs). They are simple DNA binding domain which were derived from the plant bacteria Xanthamonas. A number of TALENs are brought together to get the desired combination in order to introduce the DSB at the target site. Both TALENs and ZFNs techniques have helped the researchers to execute the gene editing in a specified manner. However, these techniques came out to be time consuming and challenging that demanded some alternate strategy for achieving the gene editing process in a precise manner.
In recent years, CRISPR - Cas9 has been introduced as a gene editing tool. Unlike ZFNs and TALENs that depends on protein engineering, CRISPR exploits short guide RNA (sgRNA) for the execution of Cas9 endonuclease to the desired target site. However, the crucial step in developing the Gene edited crops using CRISPR – Cas9 is to introduce the desired gene or CRISPR into them via gene delivery systems. The present plant transformation process exploits either Agrobacterium tumefaciens, polyethylene glycol (PEG) mediated protoplasm or biolistic as a vehicle to deliver the gene of interest. All these gene transfer methods are slow, expensive and damages the plant genome. Also, they are laborious and require resources in terms of expertise and facilities. The process is frequently inefficient and works only for a few varieties of crops.
Looking Ahead
The carbon nanoparticles are found to be valuable because of their different shapes as nanosheets, nanodots and nanotubes and size. They were first discovered in 2004. Carbon dots (CDs) can act as a carrier as they can be functionalized by PEG diamines, enabling the plasmids to interact with them electrostatically and carry into plant cells. A group of researchers, headed by Prof. Heather Whitney has used the DNA bound carbon dots coding for CRISPR machinery for genome editing. They employ the use of plant misters to spray leaves with water containing these carbon dots bound to desired gene. Hence, they were able to modify the genomes of sprayed leaf cells. Doyle et al., 2019 utilized DNA bound carbon dots as a spray to carry out the gene editing in wheat and the presence of edited gene was confirmed by transient GFP expression in the nucleus and also by DNA sequencing.
CDs system is quite useful with many advantages as it is simple, versatile, easily applicable and cost effective. The CDs are sprayed on the plants which can be easily taken up by mature tissues without damaging the plant surface. They occur naturally or can be constructed from natural non-toxic material. It is believed that the CDs system has potential to carry out the transformation in recalcitrant crop species. Hence, CDs approach seems to be convincing to transiently silence or induce the gene expression useful for plant growth and developmental research.
The exogenous application of the desired gene using carbon dots is definitely the next revolutionary step in crop protection technology. With the optimization of this technology, it will rise as a robust tool to carry out the genome editing with out harming the plant tissues. Undoubtedly, genome editing technology is an effective tool for the development of agronomic crops with desired traits and higher yields.
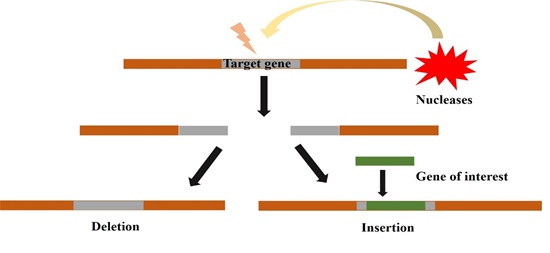
The principle behind the gene editing is the production of breaks in the site-specific double-stranded DNA which results in the activation of cellular DNA repair pathways. Some programmable enzymes, especially nucleases are responsible for initiating the double-stranded breaks (DSB) at specific genomic loci. This facilitates the removal of the existing DNA and integration of the desired DNA in the specific location (Fig1). Enzymes involved in gene editing process are also known as Molecular Scissors. In the earlier days, a special class of endonucleases called mega nucleases were exploited for gene editing. They are mobile genetic elements, derived from microbe and are capable to introduce DSB at a specific location. However, the finding of exact mega nucleases is essential to use them for gene editing which limits their use.